Blog Posts

FTC Budget Hearing Recap: Policymakers Express Concern About Agency’s Approach to M&A
Recently, Federal Trade Commission (FTC) Chair Lina Khan appeared before the House Appropriations Committee Subcommittee on Financial Services and General Government to discuss the Agency’s proposed budget for the upcoming fiscal year.

Innovations Advanced by M&A: An Innovative CAR-T Therapy for Blood Cancer
In the case of Breyanzi, the merger between Juno Therapeutics and Celgene allowed the two companies to combine their complementary resources and expertise and secure the investment required to bring a novel chimeric antigen receptor T-cell (CAR-T) therapy for advanced forms of blood cancer to patients more quickly.

Mercatus Center Policy Brief: FTC & DOJ’s Flawed Approach to M&A Impedes Early-Stage Innovation
In a recent policy brief, Satya Marar, a fellow at the Mercatus Center at George Mason University, points out the considerable harm that the Federal Trade Commission (FTC) and Department of Justice’s (DOJ) recent approach to antitrust enforcement is having on early-stage companies.

ITIF Report: Supporting R&D Investment Critical to Advance New Biopharmaceutical Breakthroughs
The United States is home to thousands of life sciences companies, with hundreds more starting every year, each competing to develop and deliver innovations to patients. Nearly 80% of these companies operate without a profit, even as the average cost of bringing a new medicine to market exceeds $2.6 billion.

Opinion: New Merger Enforcement Approach Jeopardizes Life Sciences Innovation in NJ
New Jersey has long been home to some of the world’s leading research-based biopharmaceutical companies. Many have made New Jersey a base for their global, North American, or U.S. operations. However, recent policy changes by the Federal Trade Commission and Department of Justice could jeopardize our state’s status as a health care leader.
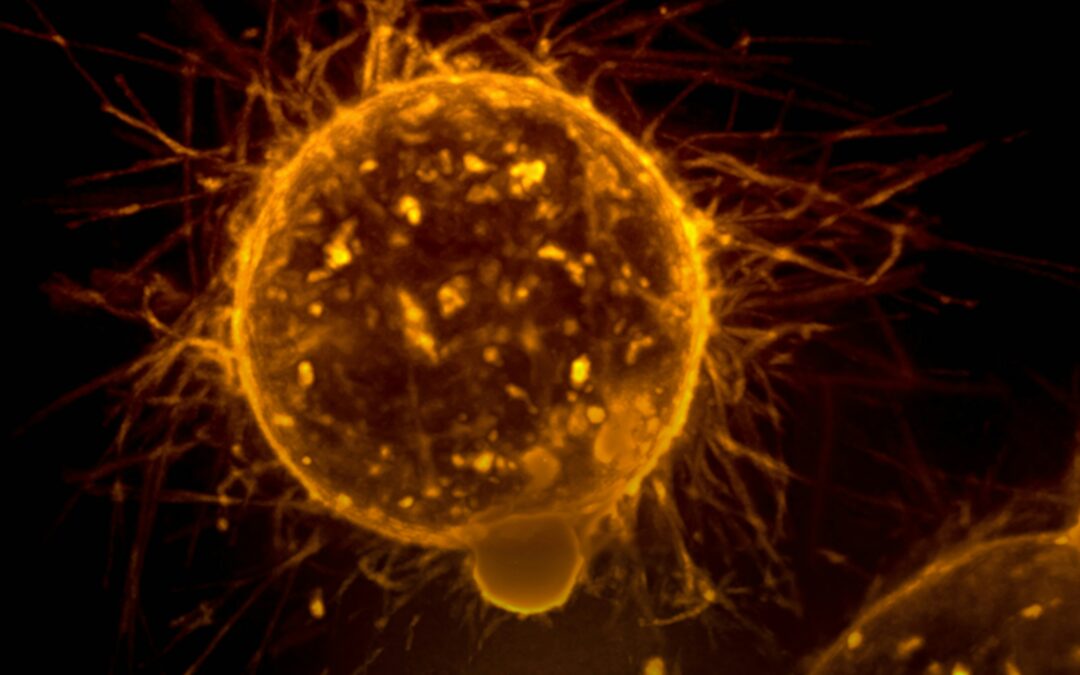
Innovations Advanced by M&A: Opdivo and Yervoy’s Substantial Impact on Cancer Treatment
Yervoy and Opdivo, two groundbreaking cancer therapies made possible through the merger of Medarex and Bristol Myers Squibb (BMS), significantly expanded the treatment options for thousands of patients with cancer worldwide.

“Fast Facts”: Early-Stage Companies Count on M&A to Bring Biopharmaceutical Breakthroughs to Patients
The United States is home to more than 2,300 life sciences companies – including more than 85 percent of the world’s small, early-stage biopharmaceutical companies – each competing to bring new treatments and cures to patients.

Innovations Advanced by M&A: The First Therapy for Pompe Disease
From the complex scientific and regulatory expertise required, to the enormous capital investment needed for state-of-the-art facilities and clinical trials, many companies cannot navigate this path alone. Mergers and acquisitions (M&A) are fundamental to shepherding medicines along the path to market, and to patients in need. Such was the case for a first-of-its-kind treatment for Pompe disease.

House Committee on Small Business Urges FTC, DOJ to Consider Consequences of Recent Merger Guidelines on Early-Stage Companies
Last week, the House Committee on Small Business wrote to the Federal Trade Commission (FTC) and Antitrust Division of the Department of Justice (DOJ), emphasizing that the merger guidelines “threaten to deter M&A transactions before they even get off the ground,” chilling a critical pathway for small companies.

‘Material Differences’ & ‘Less Burden’: FTC, DOJ Signal Forthcoming Changes to Proposed HSR Rules
Speaking to antitrust experts at the ABA’s 2024 Spring Antitrust Meeting, Andrew Forman, deputy assistant attorney general at the Department of Justice’s (DOJ) Antitrust division, suggested that the Federal Trade Commission (FTC) and DOJ’s forthcoming changes to the Hart-Scott Rodino (HSR) premerger notification rules will reflect substantive changes from their initial proposal.